CRAMS
Services - CRAMS
We have evolved to include Contract Research and Manufacturing Services as our growing business vertical. We have developed capabilities to provide a wide range of CRAMS starting from route selection / process development / optimization, analytical development, stability studies, safety studies, scale-up to technology transfer / clinical-trial manufacturing to commercial manufacturing to suit US / EU regulatory requirements. We can cost-effectively manage several complex reactions at various scales of operation, ranging from a few grams to several tonnes. For instance, we, using a proprietary bio-catalytic/ enzymatic technology availed by us, have developed innovative route for the manufacturing of key advanced intermediates.
We aim to capture value at three important parts of the CRAMS value chain through initiatives namely:
- Pre-commercial work: exclusive synthesis / custom work for innovators on NCE candidates,
- High-value technologies with significant entry barriers: manufacture of technologically challenging products, and
- Commercial manufacture of on-patent APIs and Intermediates: actively partnering in drug development efforts through custom manufacturing of APIs and Intermediates and participating in process research like scale-up of manufacturing.

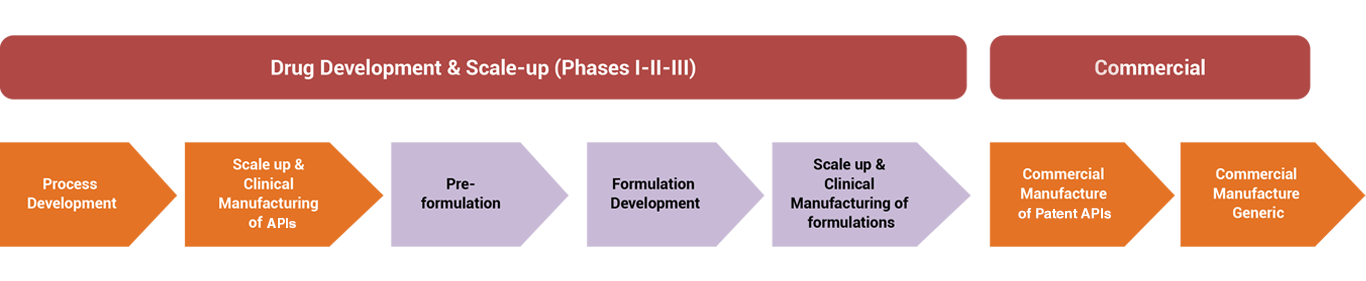
Presence of Arch Pharmalabs Limited
We are increasingly focused on moving up the value chain in the pharmaceutical services business by targeting projects from large innovator firms which can allow us to exemplify our end-to-end competence across the entire services spectrum of CRAMS. These projects typically involve customized research and manufacturing services. These are projects that form part of the drug development process, customized synthesis of drug intermediates, small-scale manufacturing of APIs for clinical trials and commercial-scale manufacture of patent protected APIs.
We focus on customised solutions based on complex technologies thereby increasing margins and customer stickiness. NCE i.e. New Chemical Entity is a chemical molecule developed by the innovator company in the early drug discovery stage, which after undergoing clinical trials could translate into a drug that could be a cure for some disease. Synthesis of NCE is the first step in the process of development of a drug. Once the synthesis of the NCE has been completed, companies have two options before them. They can either go for clinical trials on their own or license the NCE to another company. In the latter option, companies can avoid the expensive and lengthy process of clinical trials, as the licensee company would be conducting further clinical trials and subsequently launching the drug. Innovator companies adopting this model of business would be able to generate high margins as they get a huge one-time payment for the NCE apart from entering into a revenue sharing agreement with the licensee company.
We believe that executing Custom Chemical Synthesis (“CCS”) projects successfully is an opportunity to clinch commercial scale manufacturing deals with our clients. Under our CCS portfolio we have supplied key Advanced Intermediates of two pipeline drugs . We have been associated with both of these products from early phase-II trials. We have developed a wide spectrum of differentiating capabilities that we can offer in CRAMS and we believe that executing CCS projects successfully is an opportunity to clinch commercial scale manufacturing deals with our clients.
We believe CCS projects can provide opportunities in securing Contract Manufacturing contracts. We have transfer of technology under secrecy agreement. We target Contract Manufacturing of on-patent APIs / Intermediates through existing pipeline with innovator customers. We intend to leverage the capabilities that we have developed in Contract Manufacturing for generic companies and innovators to expand further. We aim to enhance visibility and consolidate relationships through Custom Synthesis and Contract Manufacturing services and become an outsourcing partner of choice for global innovator companies. We have built assets and capabilities to enter high-margin business lines through strategic acquisitions in India. Going forward we are going to carry on this strategy by increasing our presence in CRAMS and becoming a high value player with specialized product and service offerings.
